-40%
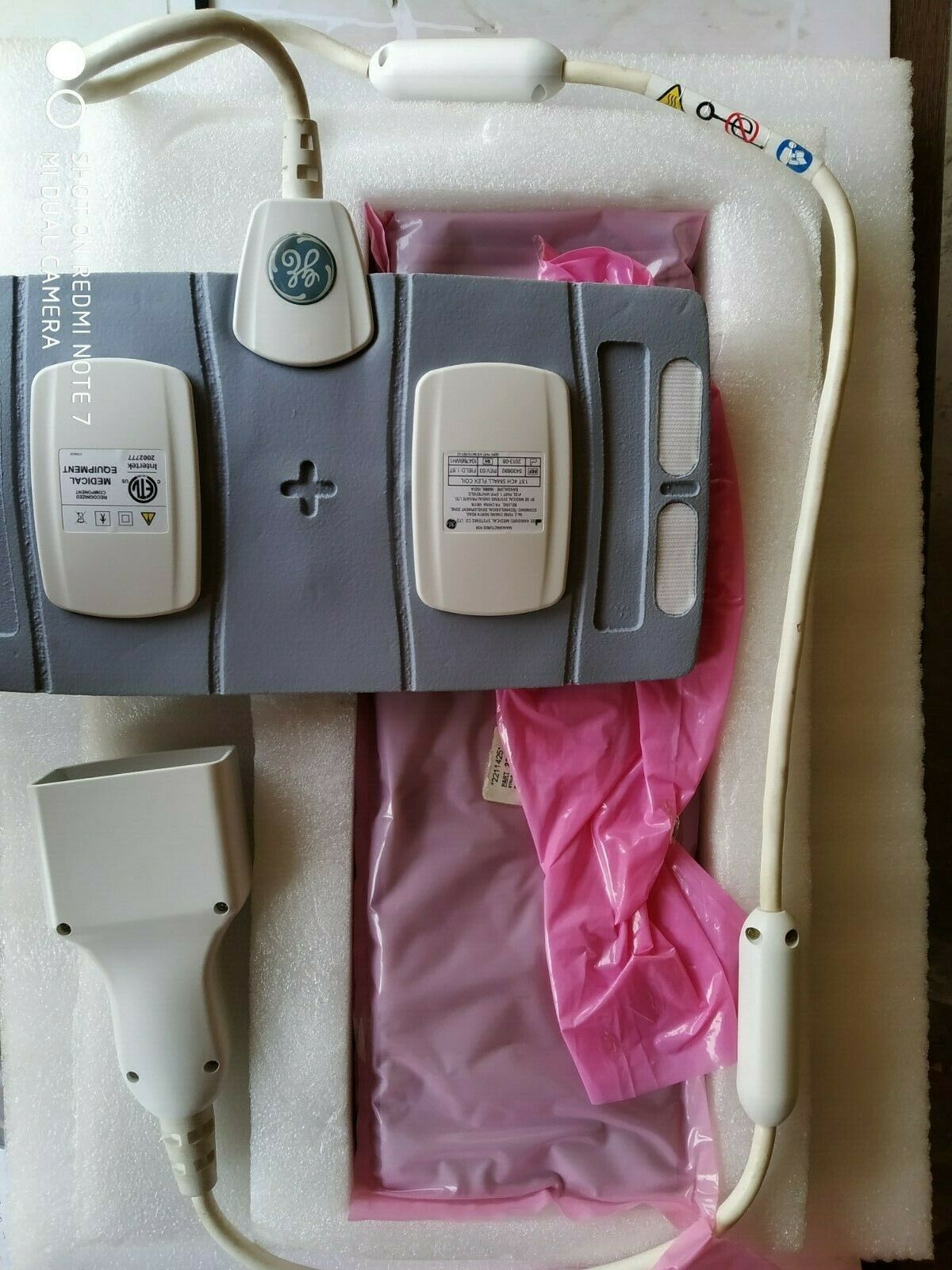
Medrad Spectris Solaris EP MR injection system complete/used
$ 10032
- Description
- Size Guide
Description
TheMedrad Spectris Solaris EP
MR injector is used for accurately injecting intravenous MR contrast media and common flushing solutions into the human vascular system for diagnostic studies in magnetic resonance imaging (MRI) procedures. The
Spectris Solaris EP
MR Injection System is a programmable dual syringe system. The system consists of two basic components, the control room unit, and the scan room unit.
3.0T compatibility.
Enhanced power management with integrated continuous battery charger.
Pressure limit control that provides enhanced control for clinicians when using additional patient access tubing like Power PICC and central lines.
6 user-programmable phases.
Color touchscreen with large numeric displays and graphics.
High-performance, 8-amp-hour battery.
Medrad Spectris Solaris EP Specifications
Control Room Unit Dimensions
Height: 10.92” (27.91 cm)
Width: 11.99” (30.46 cm)
Depth: 10.5” (26.67 cm)
Scan Room Unit Dimensions
Height: 37” (94 cm)
Width: 11.25” (28.6 cm)
Depth: 57.5” (146 cm)
Electrical
Electrical: 100-240 VAC
Requirements: 50/60Hz; 350 VA
Electrical Leakage: Unit < 100 microamperes; Patient < 10 microamperes
Syringe Volumes
Syringe A: 0.5 mL to maximum syringe volume in: 0.1 mL increments between 0.5 and 31 mL; 1 mL increments for 31 mL and above.
Syringe B: 1 mL to max. syringe volume in: 1 mL increments
Flow Rate (Programmable)
0.01 to 10 mL/s in: 0.01 mL/s increments between 0.01 and 3.1 mL/s; 0.1 mL/s increments between 3.1 and 10 mL/s
KVO
0.25 mL pulsed every
15 seconds
20 seconds
30 seconds (default)
45 seconds
60 seconds
75 seconds
System Capabilities
Pressure Safety Limit: Factory set not to exceed 325 psi (2240 kPa)
Delay: 1 to 300 seconds in 1-second increments
Pause Phase: 1 to 900 seconds in 1-second increments
Injection Capabilities: 6 phases per protocol
Storage Capacity: 32 protocols of up to 6 phases each. Protocol and user configuration memory is maintained when system power is turned off.
CONTACT ME BY SEPARATE EMAIL IF U WANT FAST SHIP. INTERNATIONAL BUYERS> Please verify domestic or international shipping address. If your item shows that it has been delivered by the post office it is no longer my responsibility.
All equipment I have for sale is inspected for functionality and signs of damage. Items may have signs of wear, scratches, etching from various facilities or stains. All items are sold as is, HOWEVER. I will accept returns for non functional items. Items must be returned. The buyer must pay shipping. Any refund will be at my discretion and paid as refund through Pay Pal.
DISCLAIMER: Regardless of the origin of the equipment, documentation provided or identification appearing upon the equipment, the equipment described and offered here is in no way certified for, recommended for, or offered for any specific use. The purchaser agrees that the seller shall not be held responsible or liable for any injuries or damages, whether incidental or consequential, associated in any way with the equipment. The purchaser, by bidding on this equipment, indicates their acknowledgment of, and agreement to the terms of this disclaimer.
FDA REGULATIONS:
The sale of this item may be subject to regulation by the U.S. Food and Drug Administration and state and local regulatory agencies. If so, do not bid on this item this item unless you are an authorized purchaser. If the item is subject to FDA regulation, I will verify your status as an authorized purchaser of this item before shipping of the item. If you have questions about legal obligations regarding sales of medical devices, you should consult with the FDA's Center for Devices and Radiological Health:
Thanks for your cooperation!